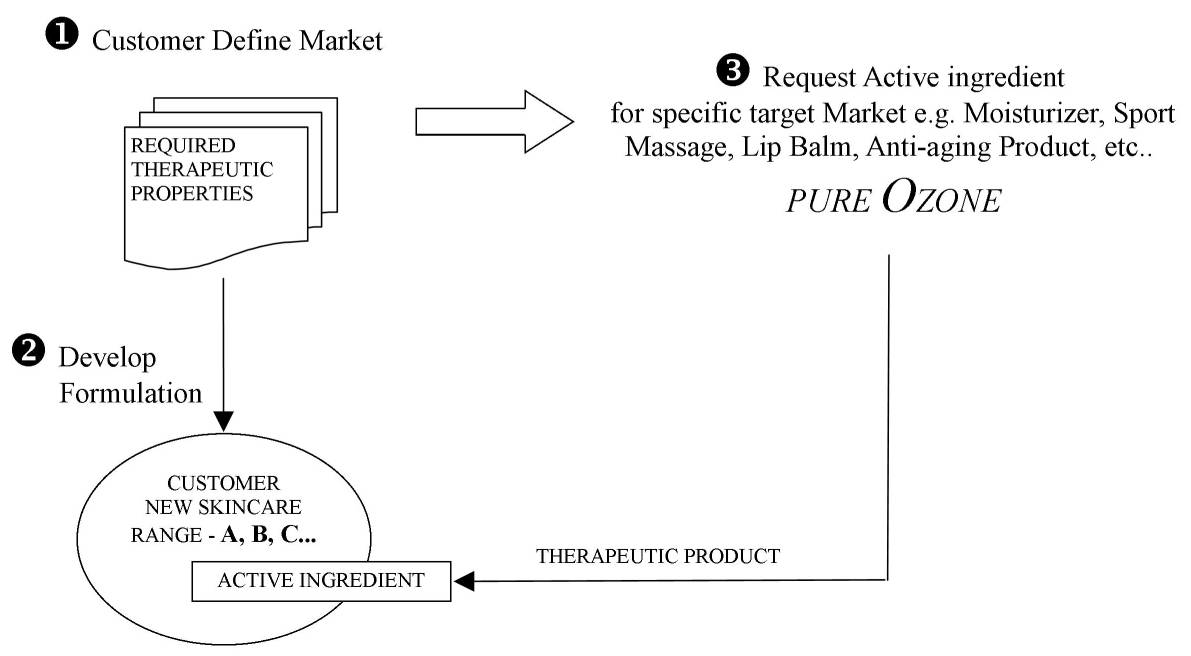
Production
In order to meet the demand of Natural active ingredients in a growing range of cosmetic produce, we follow a strategy of target-specific Oxygen-activated oil where we custom process your batch of Pure1000 , based on your target market, in order to optimize your product (see drawing below). Customers do not need to disclose any confidential information on their product and/or formulation. On this bases, Pure Ozone is proud to have customers with professional (and popular) brands on the market.
Our production process is firmly controlled and monitored to produce consistent oxygen-activated-oil production as required by our clients for specific formulation.
Product stability
While Ozone gas is utilized world wide, mainly for its powerful oxidizing and disinfection properties, it is constrained to onsite generation (sometimes at high cost) due to its inherent short life time. However when infused into vegetable oils it modifies the chemical structure and allows the ozone to be stored in a stable and active manner (See also M. Cirlini, et.al - Stability Studies of Ozonized Sunflower Oil and Enriched Cosmetics with a Dedicated Peroxide Value Determination).
Jojoba oil
Pure Ozone purchases directly from a single source, Jojoba farmer in the Semi-desert Karoo of South Africa. With this direct relationship, we can ensure the highest quality organic Jojoba at the best price. The characteristics of this particular produce is now well known by Pure Ozone, allowing for production of a consistent ozonated oil content. (see also: R. von Landsberg, "Organic" vs "Chemical-free")
Hygiene and Safety
Our protocol for Hygiene and Safety is very strict through all stages of manufacturing. For pharmaceutical-grade ozonation, absolute clean air is needed to reduce any risks of contamination during gas-infusion. Our facility situated in the picturesque Southern Cape of South Africa is free from air pollution (compare to smoky industrial areas with potentially noxious and harmful impurities), followed by dry air preparation, ultra filtration, purification, medical grade Oxygen Concentration, Ozonation, etc., etc. Only glass and ozone resistant materials are used during manufacturing with minimal contact.